Pharmacogenomics in Clinical Practice: Oncology – Online CME Course
 Online CME Course - Available until October 31, 2022
Online CME Course - Available until October 31, 2022
Course Directors | |
 | Timothy B. Curry, M.D., Ph.D. |
 | Denise M. Dupras, M.D., Ph.D. |
Focused on pharmacogenomics in oncology, this module is specifically curated from the more extensive Pharmacogenomics for Your Practice - Online certificate program. This online course provides foundational pharmacogenomics concepts, but focuses most of the content on pharmacogenomics as applied to oncology, oncology case studies, and drugs most commonly used in oncology treatment.
Target Audience
This activity has been designed for pharmacists, physicians, nurse practitioners, physician assistants, nurses, students, and other members of the health care team.
Learning Objectives
Upon conclusion of this program, participants should be able to:
- Describe what to consider when implementing pharmacogenomics in clinical practice
- Apply pharmacogenomics test results to make recommendations for an individualized medication management plan
- Identify barriers and challenges of considering pharmacogenomics in patient care
Utilization of this Mayo Clinic online (enduring materials) course does not indicate nor guarantee competence or proficiency in the performance of any procedures which may be in this course.
IMPORTANT DISCLAIMER: Some content in this course is included in other CME courses (Pharmacogenomics in Clinical Practice: Essential Concepts - Online CME Course and Pharmacogenomics in Clinical Practice: Applications in Clinical Care - Online CME Course). If you do enroll and receive credit from this course, you will not be eligible to claim it for Pharmacogenomics in Clinical Practice: Essential Concepts - Online CME Course and Pharmacogenomics in Clinical Practice: Applications in Clinical Care - Online CME Course. If duplicate credit is accidentaly claimed, it will not be valid should any auditing occur. Pleae do not hesitate to contact us with any qeustions prior to purchasing.
Additional Information
| Attachment | Size |
|---|---|
| 149.79 KB |
 | Pharmacogenomics 101: An Introduction to Foundational Concepts Christopher T. Grilli, Pharm.D., R.Ph., M.B.A. | Over the past 30 years, pharmacogenomics has been making waves throughout the medical field. What does this mean for whole-patient care? Learn the basics of genomics and genetic concepts in this lecture! |
 | PGx from a Patient Perspective: GINA & Other Important Questions Teresa M. Kruisselbrink, M.S., CGC, LCGC | Are you interested in genetic testing but are afraid of its impact on employment and health insurance? Have no fear, GINA is here! This lecture will describe the protections offered by the Genetic Information Non-Discrimination Act (GINA) and its limitations of protections. |
 | Legal Implications of Pharmacogenomics Testing Sharon C. Zehe, J.D. | With the rise of pharmacogenomics testing, providers may have concerns of the risk of malpractice with these tests. With this lecture you'll understand the elements of malpractice and apply them to PGx testing. |
 | Pharmacogenomics from the Patient Perspective: Panel Discussion | Join Dr. Darryl Chutka, Dr. Matthew Ferber, Dr. Karen Meagher, and Sharon Zehe as they discuss the exciting world of pharmacogenomics and its relation to psychiatrics! |
 | Clinical Laboratory Testing in PGx: "The Lab" 101 Ann M. Moyer, M.D., Ph.D. | So you wanna order a pharmacogenomics test. But how can the laboratory help you? Find out how the pharmacogenomics laboratory can help your practice in this lecture! |
 | Clinical Laboratory Testing in PGx: Targeted Genotyping Technology Ann M. Moyer, M.D., Ph.D. | In the past twenty years, technology has advanced to improve testing techniques and strategies. The use of preemptive testing in pharmacogenomics has shaken the world of genetic testing and continues to improve! Learn about its history and importance with Dr. Ann Moyer! |
 | Pharmacogenomics: Challenges with Transplant John Logan Black, M.D. | Hematopoietic stem cell transplant impacts the accuracy of pharmacogenomic testing. However, there are steps that can be taken to mitigate these impacts. Tune in with Dr. John Black to understand the impact and processes! |
 | Case Study: Thiopourines/TPMT and NUDT15 in Clinical Practice Edward V. Loftus, Jr., M.D. | Thiopurines have been effective for both inducing and maintaining long-term remission in Crohn's disease and ulcerative colitis. But thiopurine comes with adverse effects, such as myelosuppression. Understand thiopurines and the TPMT gene with Dr. Edward Loftus! |
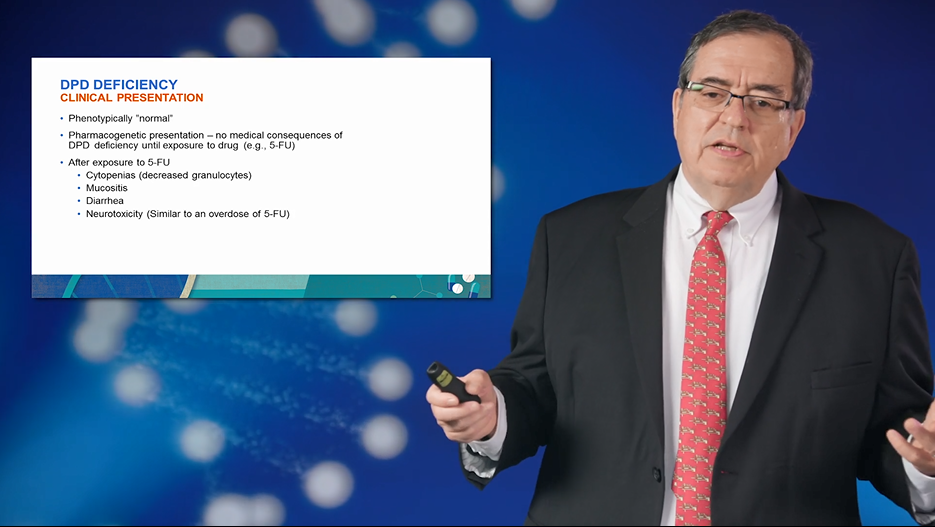 | Dihydropyrimidine Dehydrogenase (DPD) Deficiency: Implications for 5-Fluorouracil (Capecitabine) Treatment Robert B. Diasio, M.D. | Approximately 275,000 cancer patients are treated with 5-fluorouracil (5-FU) annually in the U.S. Despite its clinical importance, there are concerns with dihydropyrimidine dehydrogenase (DPD) deficiency. Join Dr. Robert Diasio to understand the relationship of DPD and 5-FU! |
 | Why is CYP2D6 Genotyping Ready for Primetime: Association of CYP2D6 Genotype and Breast Cancer Outcomes from Large Trails Matthew P. Goetz, M.D. | Dr. Matthew Goetz has notable work regarding the pharmacogenetics of tamoxifen, the CYP2D6 enzyme, and its association with breast cancer. Tune in to his primetime talk on the association of CYP2D6 genotype and breast cancer outcomes! |
 | Belinostat and UGT1A Polymorphism N. Nora Bennani, M.D. | Have you ever heard of UGT1A1 polymorphism and its effects on belinostat? Well, |
 | Oncology Caren L. Hughes, Pharm.D., R.Ph. | |
 | Pharmacogenomics in Oncology Eric J. Yancey, Pharm.D., R.Ph. | |
 | 10 Considerations for the Clinician Applying Pharmacogenomics to Practice Wayne T. Nicholson, M.D., Pharm.D. | So you want to include pharmacogenomics in your practice. Should you order a pharmacogenomics test? Should you order a single gene test or a panel? Find out with Dr. Wayne Nicholson! |
 Accreditation Statement
Accreditation Statement
In support of improving patient care, Mayo Clinic College of Medicine and Science is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC) to provide continuing education for the healthcare team.
Credit Statement(s):
AMA
Mayo Clinic College of Medicine and Science designates this enduring material for a maximum of 5.50 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
ACPE

Mayo Clinic College of Medicine and Science designates this educational activity for a maximum of 5.50 knowledge-based contact hours. Participants should only claim credit commensurate with the extent of their participation in the activity.
ACPE UAN Number: JA0000238-0000-21-088-H01-P
ANCC
Mayo Clinic College of Medicine and Science designates this activity for a maximum of 5.50 ANCC contact hours. Nurses should claim only the credit commensurate with the extent of their participation in the activity.
Other Healthcare Professionals
A record of attendance will be provided to all registrants for requesting credits in accordance with state nursing boards, specialty societies or other professional associations.
For disclosure information regarding Mayo Clinic School of Continuous Professional Development accreditation review committee member(s) and staff, please go here to review disclosures.
Available Credit
- 5.50 ACPE
- 5.50 AMA PRA Category 1 Credit™
- 5.50 ANCC
- 5.50 Attendance
Price
Cancellation and Refund Policy
View Cancellation and Refund Policy
All requests must be submitted in writing using the Contact Us Form.
Any use of this site constitutes your agreement to the Terms and Conditions of Registration.

 Facebook
Facebook X
X LinkedIn
LinkedIn Forward
Forward